lv virus production | lentivirus production workflow lv virus production Image: Illustrated plasmid map in PNG format GenBank File: Plasmid sequence . The main differences between the belts are the quality of the leather, the stitching and sometimes the replicas have LV stamping Louis Vuitton Paris made in France in the middle of the belt which doesn’t exist in the authentic one and this is an indicator of a .How to tell if Louis Vuitton is real (or fake) Bags: Check the “LOUIS VUITTON ®” inscription engraved in leather. Fake bags always have thicker text. Footwear: Verify the inscriptions on the soles. Fake shoes always have too little space in-between the text. Clothing: Look at the wash tags. A fake Louis Vuitton always has very thick prints.
0 · viral vector production cells
1 · lvs production pipeline
2 · lvs production
3 · lentivirus vector production
4 · lentivirus production workflow
5 · lentivirus production system
6 · lentivirus production process
7 · lab scale lvs production
Downloadable data -base load power identifier. Daily insights - view data in watts, amps, volts, power factor, and $. Single phase electricity monitoring - mains or branch circuits. Two (2) 200A sensors included (supports 5A - 200A) Easy non-invasive installation. Meets North American safety standards.
Introduction. This protocol can be used to produce lentivirus from a lentiviral vector transfected into 293T cells using a polyethylenimine (PEI) transfection protocol. This procedure can be modified for alternative packaging cell lines or transfection reagents.Image: Illustrated plasmid map in PNG format GenBank File: Plasmid sequence .
Image: Illustrated plasmid map in PNG format GenBank File: Plasmid sequence . LVs produced in most research laboratories contain contaminants that can .
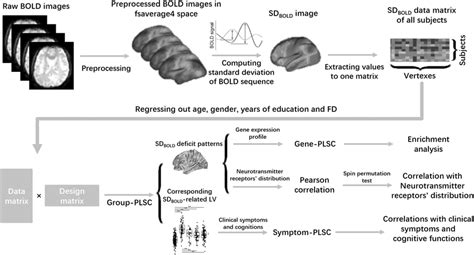
Introduction. This protocol can be used to produce lentivirus from a lentiviral vector transfected into 293T cells using a polyethylenimine (PEI) transfection protocol. This procedure can be modified for alternative packaging cell lines or transfection reagents. LVs produced in most research laboratories contain contaminants that can generate confounding effects in experimental studies. Soldi et al. describe a laboratory-scale workflow for purified LV production, highlighting enhanced gene-editing efficiency and diminished inflammatory responses.This is a comprehensive review of the individual bioprocess operations employed in LV production. We highlight the role of envelope proteins in vector design as well as their impact on the bioprocessing of lentiviral vectors.The LV-MAX Lentiviral Production System is designed to support robust lentiviral production in a variety of suspension culture vessels. You can scale up or down based on your needs for greater throughput early in discovery or for seamless and efficient clinical scale-up while maintaining high yield in a serum-free system (Figure 1) .
Lentiviral vectors (LV) have seen considerably increase in use as gene therapy vectors for the treatment of acquired and inherited diseases. This review presents the state of the art of the production of these vectors with particular emphasis on .
Introduction. Lentiviral vectors (LVs) have emerged as promising vector types and potentially a safer alternative to γ-retroviral vectors. Utilization of LVs in clinical trials has increased.
Lentivirus (LV) is an enveloped RNA virus that is characterized by its ability to incorporate viral RNA into host cell DNA. For this reason, LV vectors (LVV) are used in the cell and gene therapy field for delivery of nucleic acids into target cells both ex vivo and in vivo . Lentiviral vectors (LVs) are increasingly employed in gene and cell therapy. Standard laboratory production of LVs is not easily scalable, and research-grade LVs often contain contaminants that can interfere with downstream applications. Moreover, purified LV production pipelines have been developed mainly for costly, large-scale, clinical . Lentiviral vectors (LVs) are potent tools for the delivery of genes of interest into mammalian cells and are now commonly utilised within the growing field of cell and gene therapy for the treatment of monogenic diseases and adoptive therapies such as chimeric antigen T-cell (CAR-T) therapy.Detailed herein is a simple protocol for the production LV vectors, describing the triple transfection of an LV transfer vector and LV helper plasmids into HEK-293 cells, and the subsequent purification of virions from the cellular media.
Introduction. This protocol can be used to produce lentivirus from a lentiviral vector transfected into 293T cells using a polyethylenimine (PEI) transfection protocol. This procedure can be modified for alternative packaging cell lines or transfection reagents. LVs produced in most research laboratories contain contaminants that can generate confounding effects in experimental studies. Soldi et al. describe a laboratory-scale workflow for purified LV production, highlighting enhanced gene-editing efficiency and diminished inflammatory responses.This is a comprehensive review of the individual bioprocess operations employed in LV production. We highlight the role of envelope proteins in vector design as well as their impact on the bioprocessing of lentiviral vectors.The LV-MAX Lentiviral Production System is designed to support robust lentiviral production in a variety of suspension culture vessels. You can scale up or down based on your needs for greater throughput early in discovery or for seamless and efficient clinical scale-up while maintaining high yield in a serum-free system (Figure 1) .
Lentiviral vectors (LV) have seen considerably increase in use as gene therapy vectors for the treatment of acquired and inherited diseases. This review presents the state of the art of the production of these vectors with particular emphasis on . Introduction. Lentiviral vectors (LVs) have emerged as promising vector types and potentially a safer alternative to γ-retroviral vectors. Utilization of LVs in clinical trials has increased.Lentivirus (LV) is an enveloped RNA virus that is characterized by its ability to incorporate viral RNA into host cell DNA. For this reason, LV vectors (LVV) are used in the cell and gene therapy field for delivery of nucleic acids into target cells both ex vivo and in vivo .

Lentiviral vectors (LVs) are increasingly employed in gene and cell therapy. Standard laboratory production of LVs is not easily scalable, and research-grade LVs often contain contaminants that can interfere with downstream applications. Moreover, purified LV production pipelines have been developed mainly for costly, large-scale, clinical . Lentiviral vectors (LVs) are potent tools for the delivery of genes of interest into mammalian cells and are now commonly utilised within the growing field of cell and gene therapy for the treatment of monogenic diseases and adoptive therapies such as chimeric antigen T-cell (CAR-T) therapy.
viral vector production cells
lvs production pipeline
lvs production
lentivirus vector production
lentivirus production workflow

LED TV backlights can fail due to various reasons. One common cause is overheating, as the excessive heat can damage the LED diodes and other components. Additionally, power supply issues, such as voltage spikes or fluctuations, can also contribute to backlight failure.
lv virus production|lentivirus production workflow